Product List
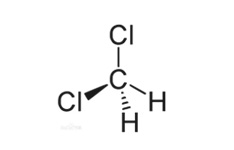
English name: Dichloromethane
Chinese alias: methylene chloride; methylene chloride; methylene chloride; methylene chloride; methylene chloride; methylene chloride; methylene chloride
CAS RN: 75-09-2
EINECS Number: 200-838-9
Molecular formula: CH2Cl2
Molecular weight: 84.93
Dichloromethane is a colorless, transparent, heavier than water, and volatile liquid with an ether-like odor and sweetness. It does not burn, but forms an explosive mixture when mixed with high concentrations of oxygen. Dichloromethane is slightly soluble in water, miscible with most common organic solvents, and miscible with other chlorine-containing solvents, ether and ethanol in any proportion. Dichloromethane is insoluble in liquid ammonia at room temperature, and can be quickly dissolved in phenol, aldehyde, ketone, glacial acetic acid, triethyl phosphate, formamide, cyclohexylamine, and ethyl acetoacetate. Pure methylene chloride has no flash point, and solvent mixtures containing equal volumes of methylene chloride and gasoline, solvent naphtha, or toluene are non-flammable, however, when methylene chloride is mixed with acetone or methanol liquids in a 10:1 ratio, Its mixed characteristic has a flash point, and the vapor and air form an explosive mixture with an explosion limit of 6.2%~15.0% (volume). Dichloromethane is the least toxic of methane chloride, and its toxicity is only 0.11% of that of carbon tetrachloride. If methylene chloride is splashed directly into the eyes, it is painful and corrosive. The vapor of dichloromethane has an anesthetic effect. When there is a serious risk of poisoning, it should be removed immediately and moved to fresh air, and some symptoms of poisoning will be relieved or disappeared without causing lasting damage.

 中文
中文